What they are,
Why they matter, and
How families can participate
Clinical Trials for CACNA1A:
At the CACNA1A Foundation, our mission is clear: to find life-changing treatments and an eventual cure for everyone living with CACNA1A-related disorders. Today, there are no FDA-approved treatments specifically for CACNA1A—but that can change with you!
Clinical trials and research studies are the bridge between a discovery in a laboratory and a treatment in a doctor’s office. By participating, our community moves from simply managing symptoms to actively testing the therapies that could change the trajectory of this disease.
We will connect our community members with trial opportunities as they become available and provide clear, unbiased educational information to support informed decision-making.
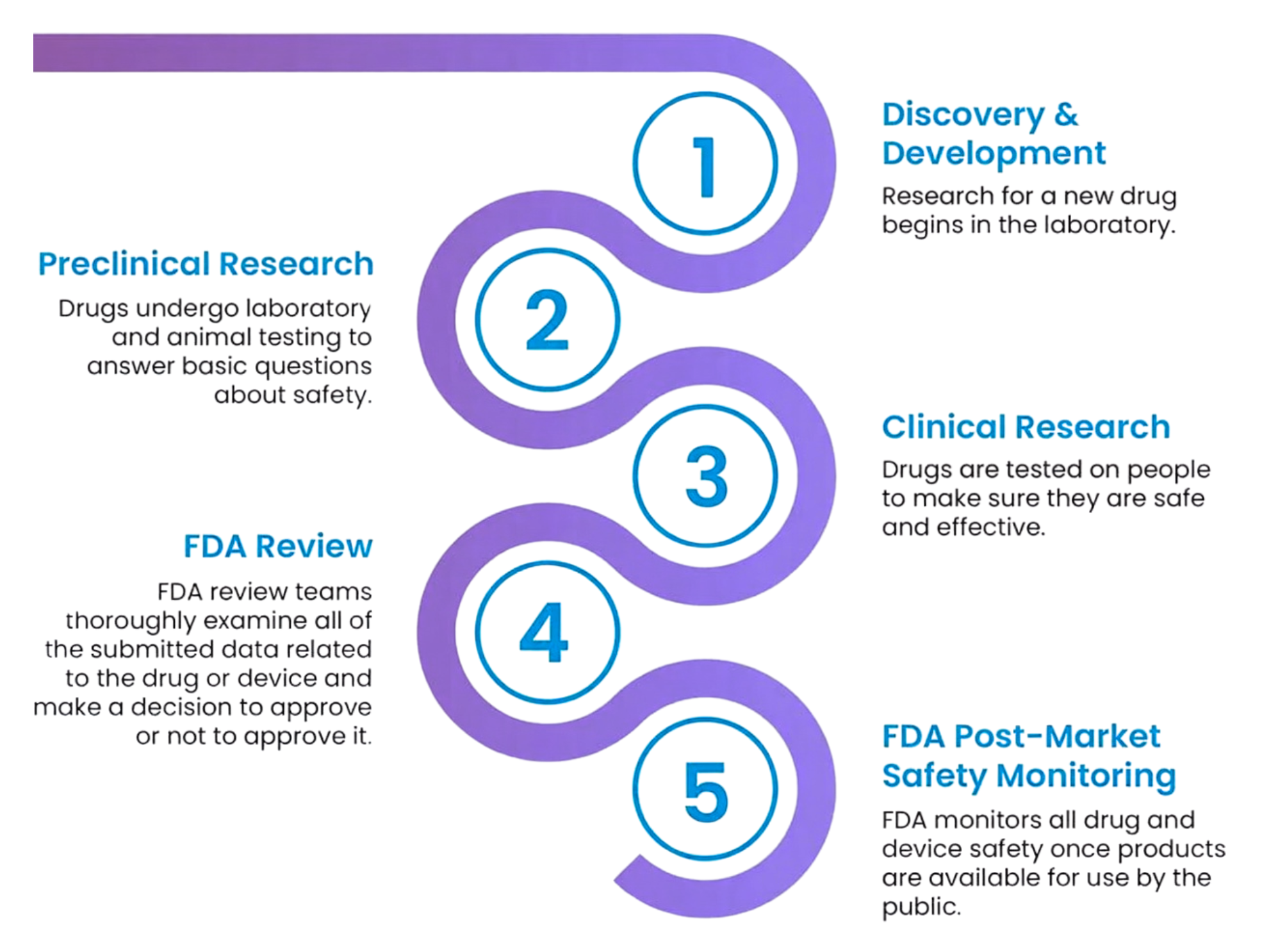
What is a Clinical Trial? - A clinical trial is a highly regulated medical research study involving human participants. Its goal is to determine whether a new treatment—such as a drug, gene therapy, or medical device—is safe and effective for use in people.
Clinical trials generally move through different phases:
Phase 1: Tests safety and dosage in a small group of people.
Phase 2: Further evaluates safety and assesses how well the treatment works (efficacy).
Phase 3: Tests the treatment in a larger group to confirm effectiveness and monitor side effects compared to standard treatments or a placebo.
Phase 4: Takes place after the FDA has approved a treatment. These studies continue to monitor safety over a longer period of time and help researchers understand how the treatment works in real-world use.
Because CACNA1A is rare, it can be challenging to enroll enough participants to advance treatments and meet FDA requirements. In rare disease research, every single participant truly matters. Without enough volunteers and data, even the most promising therapies can stall before reaching families.
We understand that participating in research is a deeply personal decision — and there is no right or wrong choice.
For those who are able and choose to participate, clinical research helps:
Advance scientific understanding of CACNA1A-related disorders
Support the development of future treatments for our community
Provide potential access to investigational therapies before they are widely available
Please note: The CACNA1A Foundation does not provide medical advice and is not affiliated with or endorsing any one life science company. Decisions regarding participation in clinical research should always be made in consultation with your personal medical team.
We encourage families to explore available options and connect directly with study teams if they have questions or would like to learn more.
Clinical Trials for CACNA1A-Targeted Treatments
1. Pivotal Study of N-acetyl-L-leucine for CACNA1A Disorders: A Phase III, Randomized, Placebo-controlled, Double-blind, Crossover Study - Enrollment should begin on September 1, 2026
Clinical Trials.Gov Identifier - NCT07221292 (https://clinicaltrials.gov/study/NCT07221292)
This is a multinational, randomized, placebo-controlled, double-blinded, crossover Phase III study that will assess the safety and efficacy of N-Acetyl-L-Leucine (IB1001) versus Placebo for the treatment of CACNA1A Disorders.
Patients will be assessed during three study periods: a baseline period (approximately 2 weeks), after which they will be randomized (1:1) to receive treatment with IB1001 or Placebo for approximately 12 weeks during the first intervention period ("Period I"). Following Period I, patients will crossover to receive the opposite treatment (IB1001 or Placebo) for approximately 12-weeks during a second intervention period ("Period II).
Patients will be assessed twice during each study period. Patients who have participated in the study may be offered the opportunity to roll over into an Extension Phase, which is planned to allow patients to have further access to IB1001.
Study Contact: Taylor Fields, tfields@intrabio.com
Clinical Trials for Epilepsy
1. A Clinical Trial for Participants With DEE to Assess Efficacy, Safety, Tolerability, and PK of Relutrigine (Emerald Study) - CURRENTLY ENROLLING
Clinical Trials.Gov Identifier - NCT07010471 (https://clinicaltrials.gov/study/NCT07010471)
A Phase 3, Randomized, Multi-Center, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Efficacy, Safety, Tolerability, and Pharmacokinetics of Relutrigine in Participants With DEE Followed by an Open-Label Extension
Choose between fully remote, in-clinic, or combined participation. If your family chooses to attend in-clinic study visits, the sponsor will pay all travel, lodging, meals, and other costs associated with study participation.
LEARN MORE ABOUT Relutrigine - This video explains the study in more detail
LEARN MORE about Praxis Precision Medicines
Study Contact: clinicaltrials@praxismedicines.com
2. A Study to Investigate LP352 (Bexicaserin) in Children and Adults With Developmental and Epileptic Encephalopathies (DEE) - CURRENTLY ENROLLING
Clinical Trials.Gov Identifier - NCT06719141 (https://clinicaltrials.gov/study/NCT06719141)
Clinical study assessing the safety of the investigational drug, Bexicaserin, and its potential to reduce the number of seizures in children and adults with developmental and epileptic encephalopathy (DEE). Bexicaserin (LP352) is a selective serotonin receptor agonist drug that is under development for the treatment of seizures in individuals with developmental epileptic encephalopathies (DEEs), including CACNA1A.
The DEEpOCEAN Study is a Phase 3 trial investigating the efficacy, safety, and tolerability of Bexicaserin in the treatment of seizures in children and adults with DEEs. Lundbeck/Longboard Pharmaceuticals is sponsoring the trial.
80 sites in 17 countries, including over two dozen in the US
Please visit these websites for more information and to see if your loved one qualifies to take part in the study:
Rest of World: https://deepdeestudy.global/
Study Contact: https://deepdeestudy.com/ , email: DEEpStudies@Lundbeck.com